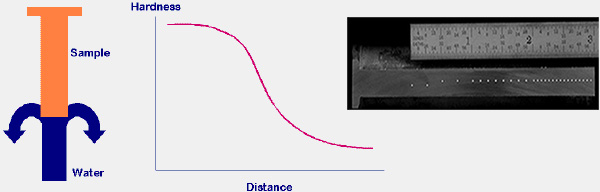
The Jominy End Quench Test measures Hardenability of steels. Hardenability is a measure of the capacity of a steel to be hardened in
depth when quenched from its austenitizing temperature. Hardenability of a steel should not be confused with the hardness of a steel. The Hardness of
a steel refers to its ability to resist deformation when a load is applied, whereas hardenability refers to its ability to be hardened to a particular
depth under a particular set of conditions. Information gained from this test is necessary in selecting the proper combination of alloy steel and heat
treatment to minimize thermal stresses and distortion when manufacturing components of various sizes.
To perform the Jominy Test: First, a sample specimen cylinder either 100mm in length and 25mm in diameter, or alternatively, 102mm by
25.4mm is obtained. Second, the steel sample is normalized to eliminate differences in microstructure due to previous forging, and then it is
austenitised. This is usually at a temperature of 800 to 900°C. Next, the specimen is rapidly transferred to the test machine, where it is held
vertically and sprayed with a controlled flow of water onto one end of the sample. This cools the specimen from one end, simulating the effect of
quenching a larger steel component in water. Because the cooling rate decreases as one moves further from the quenched end, you can measure the effects
of a wide range of cooling rates from vary rapid at the quenched end to air cooled at the far end.
Next, the specimen is ground flat along its length to a depth of .38mm (15 thousandths of an inch) to remove decarburized material.
The hardness is measured at intervals along its length beginning at the quenched end. For alloyed steels an interval of 1.5mm is commonly used where
as with carbon steels an interval of .75mm is typically employed.
And finally the Rockwell or Vickers hardness values are plotted versus distance from the quenched end.
The Jominy Test data illustrates the effect of alloying and microstructure on the hardenability of steels. Commonly used elements that affect
the hardenability of steel are carbon, boron, Chromium, Manganese, Molybdenum, Silicon, and Nickel.
Carbon is primarily a hardening agent in steel, although to a small degree it also increases hardenability by slowing the formation of pearlite
and ferrite. But this affect is too small to be used as a control factor for hardenability.
Boron can be an effective alloy for improving hardenability at levels as low as .0005%. Boron is most effective in steels of 0.25% Carbon
or less. Boron combines readily with both Nitrogen and Oxygen and in so doing its effect on hardenability is sacrificed.
Therefore Boron must remain in solution in order to be affective. Aluminum and Titanium are commonly added as "gettering" agents
to react with the Oxygen and Nitrogen in preference to the Boron.
Slowing the phase transformation of austenite to ferrite and pearlite increases the hardenability of steels. Chromium, Molybdenum, Manganese,
Silicon, Nickel and Vanadium all effect the hardenability of steels in this manner. Chromium, Molybdenum and Manganese being used most often.